Scientists have pioneered a groundbreaking method to combat snake venom using newly designed proteins, offering hope for more effective, accessible, and affordable antivenom solutions.
By utilizing advanced computational techniques and deep learning, this innovative approach has already shown promising results in neutralizing deadly toxins, potentially transforming antivenom development, and offering new strategies for tackling other neglected diseases.
Breakthrough in Antivenom Research
Scientists have designed new proteins — unlike any found in nature — that can neutralize some of the most toxic components of snake venom. Using advanced deep learning and computational methods, researchers have developed these proteins with the potential to create safer, more affordable, and widely accessible treatments compared to existing antivenoms.
Each year, over 2 million people suffer from snakebites, with more than 100,000 deaths and 300,000 cases of severe complications, including limb deformities, amputations, and other long-term disabilities, according to the World Health Organization. The highest burden of snakebites is seen in regions such as Sub-Saharan Africa, South Asia, Papua New Guinea, and Latin America, where access to effective treatment is often limited.
Advancements in Computational Biology
This groundbreaking computational biology research, aimed at improving antivenom therapies, was led by scientists from the UW Medicine Institute for Protein Design and the Technical University of Denmark. Their findings were published in Nature on January 15.
The lead author of the paper is Susana Vazquez Torres of the Department of Biochemistry at the UW School of Medicine and the UW Graduate Program in Biological Physics. Her hometown is Querétaro, Mexico, which is located near viper and rattlesnake habitats. Her professional goal is to invent new drugs for neglected diseases and injuries, including snakebites.

The Challenge of Elapid Snakebites
Her research team, which also included international experts in snakebite research, drugs and diagnostics, and tropical medicine from the United Kingdom and Denmark, concentrated their attention on finding ways to neutralize venom gathered from certain elapids. Elapids are a large group of poisonous snakes, among them cobras and mambas, that live in the tropics and subtropics.
Most elapid species have two small fangs shaped like shallow needles. During a tenacious bite, the fangs can inject venom from glands at the back of the snake’s jaw. Among the venom’s components are potentially lethal three-finger toxins. These chemicals damage bodily tissues by killing cells. More seriously, by interrupting signals between nerves and muscles, three-finger toxins can cause paralysis and death.
Limitations of Current Treatments
At present, venomous snakebites from elapids are treated with antibodies taken from the plasma of animals that have been immunized against the snake toxin. Producing the antibodies is costly, and they have limited effectiveness against three-finger toxins. This treatment can also have serious side effects, including causing the patient to go into shock or respiratory distress.
“Efforts to try to develop new drugs have been slow and laborious,” noted Vazquez Torres.
Innovations in Protein Design
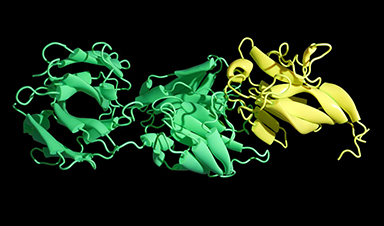
The researchers used deep learning computational methods to try to speed the discovery of better treatments. They created new proteins that interfered with the neurotoxic and cell-destroying properties of the three-finger toxin chemicals by binding with them.
Through experimental screening, the scientists obtained designs that generated proteins with thermal stability and high binding affinity. The actual synthesized proteins were almost a complete match at the atomic level with the deep-learning computer design.
In lab dishes, the designed proteins effectively neutralized all three of the subfamilies of three-finger toxins tested. When given to mice, the designed proteins protected the animals from what could have been a lethal neurotoxin exposure.
Promising Results and Future Directions
Designed proteins have key advantages. They could be manufactured with consistent quality through recombinant DNA technologies instead of by immunizing animals. (Recombinant DNA technologies in this case refer to the lab methods the scientists employed to take a computationally designed blueprint for a new protein and synthesize that protein.)
Also, the new proteins designed against snake toxins are small, compared to antibodies. Their smaller size might allow for greater penetration into tissues to quickly counteract the toxins and reduce damage.
Expanding the Potential of Computational Design
In addition to opening new avenues to develop antivenoms, the researchers think computational design methods could be used to develop other antidotes. Such methods also might be used to discover medications for undertreated illnesses that affect countries with significantly limited scientific research resources.
“Computational design methodology could substantially reduce the costs and resource requirements for development of therapies for neglected tropical diseases,” the researchers noted.
Explore Further: AI Triumphs Over Venom: Revolutionary Snakebite Antidotes Unveiled
Reference: “De novo designed proteins neutralize lethal snake venom toxins” by Susana Vázquez Torres, Melisa Benard Valle, Stephen P. Mackessy, Stefanie K. Menzies, Nicholas R. Casewell, Shirin Ahmadi, Nick J. Burlet, Edin Muratspahić, Isaac Sappington, Max D. Overath, Esperanza Rivera-de-Torre, Jann Ledergerber, Andreas H. Laustsen, Kim Boddum, Asim K. Bera, Alex Kang, Evans Brackenbrough, Iara A. Cardoso, Edouard P. Crittenden, Rebecca J. Edge, Justin Decarreau, Robert J. Ragotte, Arvind S. Pillai, Mohamad Abedi, Hannah L. Han, Stacey R. Gerben, Analisa Murray, Rebecca Skotheim, Lynda Stuart, Lance Stewart, Thomas J. A. Fryer, Timothy P. Jenkins and David Baker, 15 January 2025, Nature.
DOI: 10.1038/s41586-024-08393-x
The senior researchers on the project to design protein treatments for elapid snakebites were Timothy J. Perkins at the Technical University of Denmark and David Baker of the UW Medicine Institute for Protein Design and the Howard Hughes Medical Institute. Baker is a professor of biochemistry at the UW School of Medicine.
The University of Washington has submitted a provisional U.S. patent application for the design and composition of the proteins created in this study.
News
AI Outperforms Physicians in Real-World Urgent Care Decisions, Study Finds
The study, conducted at the virtual urgent care clinic Cedars-Sinai Connect in LA, compared recommendations given in about 500 visits of adult patients with relatively common symptoms – respiratory, urinary, eye, vaginal and dental. [...]
Challenging the Big Bang: A Multi-Singularity Origin for the Universe
In a study published in the journal Classical and Quantum Gravity, Dr. Richard Lieu, a physics professor at The University of Alabama in Huntsville (UAH), which is a part of The University of Alabama System, suggests that [...]
New drug restores vision by regenerating retinal nerves
Vision is one of the most crucial human senses, yet over 300 million people worldwide are at risk of vision loss due to various retinal diseases. While recent advancements in retinal disease treatments have [...]
Shingles vaccine cuts dementia risk by 20%, new study shows
A shingles shot may do more than prevent rash — it could help shield the aging brain from dementia, according to a landmark study using real-world data from the UK. A routine vaccine could [...]
AI Predicts Sudden Cardiac Arrest Days Before It Strikes
AI can now predict deadly heart arrhythmias up to two weeks in advance, potentially transforming cardiac care. Artificial intelligence could play a key role in preventing many cases of sudden cardiac death, according to [...]
NanoApps Medical is a Top 20 Feedspot Nanotech Blog
There is an ocean of Nanotechnology news published every day. Feedspot saves us a lot of time and we recommend it. We have been using it since 2018. Feedspot is a freemium online RSS [...]
This Startup Says It Can Clean Your Blood of Microplastics
This is a non-exhaustive list of places microplastics have been found: Mount Everest, the Mariana Trench, Antarctic snow, clouds, plankton, turtles, whales, cattle, birds, tap water, beer, salt, human placentas, semen, breast milk, feces, testicles, [...]
New Blood Test Detects Alzheimer’s and Tracks Its Progression With 92% Accuracy
The new test could help identify which patients are most likely to benefit from new Alzheimer’s drugs. A newly developed blood test for Alzheimer’s disease not only helps confirm the presence of the condition but also [...]
The CDC buried a measles forecast that stressed the need for vaccinations
This story was originally published on ProPublica, a nonprofit newsroom that investigates abuses of power. Sign up to receive our biggest stories as soon as they’re published. ProPublica — Leaders at the Centers for Disease Control and Prevention [...]
Light-Driven Plasmonic Microrobots for Nanoparticle Manipulation
A recent study published in Nature Communications presents a new microrobotic platform designed to improve the precision and versatility of nanoparticle manipulation using light. Led by Jin Qin and colleagues, the research addresses limitations in traditional [...]
Cancer’s “Master Switch” Blocked for Good in Landmark Study
Researchers discovered peptides that permanently block a key cancer protein once thought untreatable, using a new screening method to test their effectiveness inside cells. For the first time, scientists have identified promising drug candidates [...]
AI self-cloning claims: A new frontier or a looming threat?
Chinese scientists claim that some AI models can replicate themselves and protect against shutdown. Has artificial intelligence crossed the so-called red line? Chinese researchers have published two reports on arXiv claiming that some artificial [...]
New Drug Turns Human Blood Into Mosquito-Killing Weapon
Nitisinone, a drug for rare diseases, kills mosquitoes when present in human blood and may become a new tool to fight malaria, offering longer-lasting, environmentally safer effects than ivermectin. Controlling mosquito populations is a [...]
DNA Microscopy Creates 3D Maps of Life From the Inside Out
What if you could take a picture of every gene inside a living organism—not with light, but with DNA itself? Scientists at the University of Chicago have pioneered a revolutionary imaging technique called volumetric DNA microscopy. It builds [...]
Scientists Just Captured the Stunning Process That Shapes Chromosomes
Scientists at EMBL have captured how human chromosomes fold into their signature rod shape during cell division, using a groundbreaking method called LoopTrace. By observing overlapping DNA loops forming in high resolution, they revealed that large [...]
Bird Flu Virus Is Mutating Fast – Scientists Say Our Vaccines May Not Be Enough
H5N1 influenza is evolving rapidly, weakening the effectiveness of existing antibodies and increasing its potential threat to humans. Scientists at UNC Charlotte and MIT used high-performance computational modeling to analyze thousands of viral protein-antibody interactions, revealing [...]