The element actinium was first discovered at the turn of the 20th century, but even now, nearly 125 years later, researchers still don’t have a good grasp on the metal’s chemistry. That’s because actinium is only available in extremely small amounts and working with the radioactive material requires special facilities. But to improve emerging cancer treatments using actinium, researchers will need to better understand how the element binds with other molecules.
In a study led by the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab), researchers grew crystals containing actinium and studied the compound’s atomic structure. While elements often behave similarly to their lighter cousins on the periodic table, researchers were surprised to find that the actinium behaved differently than predicted by looking at its counterpart, lanthanum.
“There’s a breadth of applications for these elements, from nuclear energy to medicine to national security, but if we don’t know how they behave, that inhibits the progress we can make,” said Jen Wacker, first author of the paper published in Nature Communications and a chemist at Berkeley Lab.
“We’re seeing that this work is necessary to really understand the complexity of these radioactive elements, because in a lot of cases, using their surrogates is not sufficient to understand their chemistry.”
One area of interest is in using an isotope of actinium (actinium-225) in a cancer treatment method called targeted alpha therapy (TAT), which has shown promise in clinical trials. The TAT method uses biological delivery systems such as peptides or antibodies to move the radioactive element to the cancer site.
When the actinium decays, it releases energetic particles that travel a short distance, destroying the nearby cancer cells but sparing healthy tissue further away.

“There’s a movement to design better delivery systems to get the actinium to particular cells and keep it there,” said Rebecca Abergel, a UC Berkeley associate professor of nuclear engineering and of chemistry who leads the Heavy Element Chemistry Group at Berkeley Lab.
“If we can engineer proteins to bind the actinium with a really high affinity, and either be fused with an antibody or serve as the targeting protein, that would really enable new ways to develop radiopharmaceuticals.”
Researchers used a novel approach to grow the crystals using only 5 micrograms of pure actinium—roughly one tenth the weight of a grain of salt, and invisible to the naked eye. They first purified the actinium through a complex filtration process that removed other elements and chemical impurities.
They then bound the actinium to a metal-trapping molecule called a ligand and enveloped the bundle inside of a protein isolated and purified by Roland Strong’s team at the Fred Hutchinson Cancer Center, building a “macromolecular scaffold.”
The crystals, grown over a week inside of the Heavy Element Research Laboratory, were then cryocooled in liquid nitrogen and illuminated with X-rays at Berkeley Lab’s Advanced Light Source (ALS). The X-rays revealed the compound’s 3D structure and showed how actinium interacted with surrounding atoms. It is the first single-crystal X-ray structure reported for actinium
“I’ve been working in crystallography for 40 years and seen a lot of things, and the method the team is using is unique and provides details we couldn’t get in the past,” said Marc Allaire, a scientist in Berkeley Lab’s Molecular Biophysics and Integrated Bioimaging Division and head of the Berkeley Center for Structural Biology team at the ALS.
“To the best of my knowledge, Berkeley Lab is the only place in the world where we do this kind of study and measure radioactive protein crystals.”
-
Joshua Woods and Appie Peterson measure a small sample of actinium. Credit: Marilyn Sargent/Berkeley Lab -
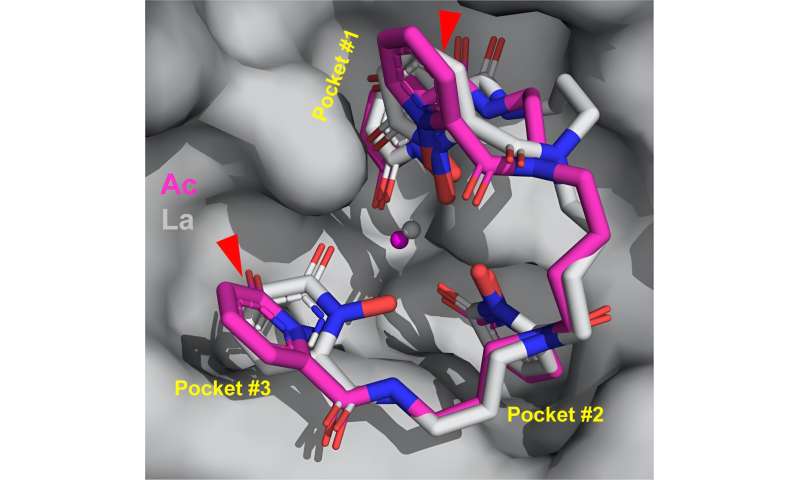
This rendering shows the structure of how actinium (magenta) binds with other molecules. Red triangles point out how the arrangement differs from actinium’s lighter counterpart, lanthanum (gray). The stick structure of the binding molecule (the ligand) is surrounded by pockets in the protein. Credit: Jen Wacker/Berkeley Lab
In this work, scientists used actinium-227, the longest-lived isotope of the element. Future studies will explore actinium-225 (the preferred isotope for targeted alpha therapy) to look for other changes in how the metal binds. Researchers are also interested in pairing actinium with different proteins to learn more about the structures it forms.
“This is very fundamental science that is part of our core program in understanding the chemistry of heavy elements,” Abergel said.
“We’ve achieved a really technically difficult experimental method that pushes the boundaries of isotope chemistry and lets us gain a better understanding of this element. It hopefully will enable us and others to develop better systems that are useful for targeted alpha therapy.”
More information: Jennifer N. Wacker et al, Actinium chelation and crystallization in a macromolecular scaffold, Nature Communications (2024). DOI: 10.1038/s41467-024-50017-5
Journal information: Nature Communications
Provided by Lawrence Berkeley National Laboratory
News
Studies detail high rates of long COVID among healthcare, dental workers
Researchers have estimated approximately 8% of Americas have ever experienced long COVID, or lasting symptoms, following an acute COVID-19 infection. Now two recent international studies suggest that the percentage is much higher among healthcare workers [...]
Melting Arctic Ice May Unleash Ancient Deadly Diseases, Scientists Warn
Melting Arctic ice increases human and animal interactions, raising the risk of infectious disease spread. Researchers urge early intervention and surveillance. Climate change is opening new pathways for the spread of infectious diseases such [...]
Scientists May Have Found a Secret Weapon To Stop Pancreatic Cancer Before It Starts
Researchers at Cold Spring Harbor Laboratory have found that blocking the FGFR2 and EGFR genes can stop early-stage pancreatic cancer from progressing, offering a promising path toward prevention. Pancreatic cancer is expected to become [...]
Breakthrough Drug Restores Vision: Researchers Successfully Reverse Retinal Damage
Blocking the PROX1 protein allowed KAIST researchers to regenerate damaged retinas and restore vision in mice. Vision is one of the most important human senses, yet more than 300 million people around the world are at [...]
Differentiating cancerous and healthy cells through motion analysis
Researchers from Tokyo Metropolitan University have found that the motion of unlabeled cells can be used to tell whether they are cancerous or healthy. They observed malignant fibrosarcoma [...]
This Tiny Cellular Gate Could Be the Key to Curing Cancer – And Regrowing Hair
After more than five decades of mystery, scientists have finally unveiled the detailed structure and function of a long-theorized molecular machine in our mitochondria — the mitochondrial pyruvate carrier. This microscopic gatekeeper controls how [...]
Unlocking Vision’s Secrets: Researchers Reveal 3D Structure of Key Eye Protein
Researchers have uncovered the 3D structure of RBP3, a key protein in vision, revealing how it transports retinoids and fatty acids and how its dysfunction may lead to retinal diseases. Proteins play a critical [...]
5 Key Facts About Nanoplastics and How They Affect the Human Body
Nanoplastics are typically defined as plastic particles smaller than 1000 nanometers. These particles are increasingly being detected in human tissues: they can bypass biological barriers, accumulate in organs, and may influence health in ways [...]
Measles Is Back: Doctors Warn of Dangerous Surge Across the U.S.
Parents are encouraged to contact their pediatrician if their child has been exposed to measles or is showing symptoms. Pediatric infectious disease experts are emphasizing the critical importance of measles vaccination, as the highly [...]
AI at the Speed of Light: How Silicon Photonics Are Reinventing Hardware
A cutting-edge AI acceleration platform powered by light rather than electricity could revolutionize how AI is trained and deployed. Using photonic integrated circuits made from advanced III-V semiconductors, researchers have developed a system that vastly [...]
A Grain of Brain, 523 Million Synapses, Most Complicated Neuroscience Experiment Ever Attempted
A team of over 150 scientists has achieved what once seemed impossible: a complete wiring and activity map of a tiny section of a mammalian brain. This feat, part of the MICrONS Project, rivals [...]
The Secret “Radar” Bacteria Use To Outsmart Their Enemies
A chemical radar allows bacteria to sense and eliminate predators. Investigating how microorganisms communicate deepens our understanding of the complex ecological interactions that shape our environment is an area of key focus for the [...]
Psychologists explore ethical issues associated with human-AI relationships
It's becoming increasingly commonplace for people to develop intimate, long-term relationships with artificial intelligence (AI) technologies. At their extreme, people have "married" their AI companions in non-legally binding ceremonies, and at least two people [...]
When You Lose Weight, Where Does It Actually Go?
Most health professionals lack a clear understanding of how body fat is lost, often subscribing to misconceptions like fat converting to energy or muscle. The truth is, fat is actually broken down into carbon [...]
How Everyday Plastics Quietly Turn Into DNA-Damaging Nanoparticles
The same unique structure that makes plastic so versatile also makes it susceptible to breaking down into harmful micro- and nanoscale particles. The world is saturated with trillions of microscopic and nanoscopic plastic particles, some smaller [...]
AI Outperforms Physicians in Real-World Urgent Care Decisions, Study Finds
The study, conducted at the virtual urgent care clinic Cedars-Sinai Connect in LA, compared recommendations given in about 500 visits of adult patients with relatively common symptoms – respiratory, urinary, eye, vaginal and dental. [...]