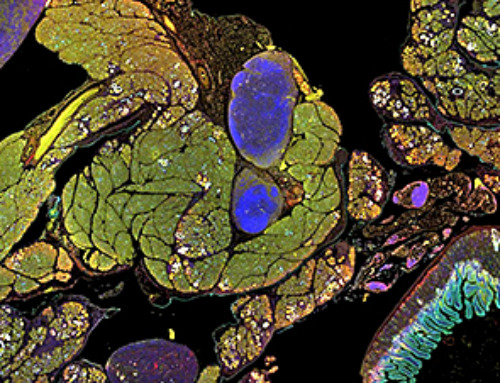
Findings suggest that PER3 gene variants prevent adrenal adaptation to winter daylight, leading to serotonin disruption and depression-like behaviors.
A recent study in Nature Metabolism used humanized mice with modified PERIOD3 gene variants (P415A and H417R) to explore the genetic role in winter seasonal affective disorder (SAD). Male mice exposed to short, winter-like daylight showed SAD-like behaviors, validating them as potential models for SAD research.
The study revealed that these gene variants increase corticosterone biosynthesis and disrupt HPA axis regulation, leading to elevated glucocorticoid signaling. This signaling represses Tryptophan hydroxylase 2 (Tph2), resulting in depression-like behaviors.
Study background
Several human physiological processes and clinical conditions exhibit seasonal rhythms, often linked to increases in pathogen or vector populations (in the case of transmissible diseases) or changes in environmental cues (such as mood and physiological shifts due to jetlag).
A growing body of research describes seasonal trends in psychiatric disorders, with conditions like depression, schizophrenia, and suicidal tendencies peaking during specific times of the year and subsiding during others.
The most well-documented of these trends is “winter seasonal affective disorder” (SAD), a relatively rare condition marked by the predictable onset of depressive episodes in autumn and winter, with remission in spring and summer.
SAD affects an estimated 1-10% of the population, with symptoms that can persist for up to 40% of the year, causing significant distress for patients and their families. Previous research has suggested that circadian misalignments and associated changes in monoamine neurotransmitters may play a role in SAD, but the precise mechanisms and potential genetic factors remain unconfirmed.
About the study
In their previous work, the present study group identified genetic variants of the PERIOD3 (PER3) gene that demonstrate advanced sleep patterns and seasonal mood alterations reminiscent of SAD. Called ‘P415A’ and ‘H417R’, these variants could hold the key to understanding SAD and form the basis of future therapeutic interventions against the debilitating condition.
The study used humanized mice (C57BL/6J and B6.129) genetically modified to express P415A and H417R for experimental procedures. Case (P415A or H417R) and control (wild type [WT]) mice were raised under varying daily light and dark cycles to simulate winter photophases. Advanced biochemical assays (immunoblotting, reverse transcription polymerase chain reaction [RT-PCR], plasma corticosterone assessments) were used to monitor both cohorts’ responses to photoperiod alterations.
Social interaction tests, tail suspension tests (TSTs), and forced swim tests (FST) were used to assess mood and behavioral alterations during experimental exposures (varying photoperiods).
Once the study had established the association between SAD and the genetic variants under study, Fluoxetine hydrochloride was administered to evaluate the mechanisms governing these associations.
Fluoxetine hydrochloride functions as a serotonin uptake inhibitor and helps reveal the importance of neurotransmitter concentrations and signaling under these conditions.
Study findings
Comparisons between case and control mice exposure to 4 h light-20 h dark (4L20D; “winter”) and 12 h light-12 h dark (12L12D; “normal”) photoperiods revealed substantial differences between carriers of the WT PER3 gene and those with the P415A or H417R variants.
Under 4L20D conditions, case mice were observed to significantly underperform controls in both TST and FST tests, displaying extended latency and immobilization across both examinations. These observations are nearly identical to the behavioral responses of SAD patients.
Social experiments revealed similar trends. Cases exposed to winter photoperiods displayed SAD-like isolation tendencies absent in controls.
These findings verify the humanized murine models used herein as apt representations of SAD across both physiology and behavior. Furthermore, these changes were reversed when mice were returned to 12L12D photoperiods.
Biochemical assays, in contrast, reported unexpected increases in corticosteroid concentrations.
Unlike previous studies, which regularly observed decreases or no changes in corticosteroid quantities, mice with P415A or H417R unregulated their neurotransmitter concentrations compared to controls, which downregulated corticosteroid production.
Fluoxetine hydrochloride drug administration was observed to rescue case mice both from corticosteroid upregulation and holistic SAD symptoms. Surgical removal of the adrenal glands (adrenalectomy) produced similar results.
Conclusions
The present study presents one of the first pieces of evidence of a genetic underpinning (herein, variants of the PER3 gene) governing periodic cyclic psychiatric states.
Experiments on humanized murine model systems revealed that P415A and H417R variants unregulated (rather than downregulated) corticosterone production, thereby disrupting normal stress responses and triggering situation-dependent depression.
These findings advance our understanding of the pathophysiology of SAD, provide a model system for future investigation (humanized mice), and highlight corticosterone modulation as a potential therapeutic intervention against human SAD.
- Gao, Q., Tang, Z., Wang, H. et al. (2024) Human PERIOD3 variants lead to winter depression-like behaviours via glucocorticoid signalling. Nat Metab. doi:10.1038/s42255-024-01163-z.https://www.nature.com/articles/s42255-024-01163-z
News
Studies detail high rates of long COVID among healthcare, dental workers
Researchers have estimated approximately 8% of Americas have ever experienced long COVID, or lasting symptoms, following an acute COVID-19 infection. Now two recent international studies suggest that the percentage is much higher among healthcare workers [...]
Melting Arctic Ice May Unleash Ancient Deadly Diseases, Scientists Warn
Melting Arctic ice increases human and animal interactions, raising the risk of infectious disease spread. Researchers urge early intervention and surveillance. Climate change is opening new pathways for the spread of infectious diseases such [...]
Scientists May Have Found a Secret Weapon To Stop Pancreatic Cancer Before It Starts
Researchers at Cold Spring Harbor Laboratory have found that blocking the FGFR2 and EGFR genes can stop early-stage pancreatic cancer from progressing, offering a promising path toward prevention. Pancreatic cancer is expected to become [...]
Breakthrough Drug Restores Vision: Researchers Successfully Reverse Retinal Damage
Blocking the PROX1 protein allowed KAIST researchers to regenerate damaged retinas and restore vision in mice. Vision is one of the most important human senses, yet more than 300 million people around the world are at [...]
Differentiating cancerous and healthy cells through motion analysis
Researchers from Tokyo Metropolitan University have found that the motion of unlabeled cells can be used to tell whether they are cancerous or healthy. They observed malignant fibrosarcoma cells and [...]
This Tiny Cellular Gate Could Be the Key to Curing Cancer – And Regrowing Hair
After more than five decades of mystery, scientists have finally unveiled the detailed structure and function of a long-theorized molecular machine in our mitochondria — the mitochondrial pyruvate carrier. This microscopic gatekeeper controls how [...]
Unlocking Vision’s Secrets: Researchers Reveal 3D Structure of Key Eye Protein
Researchers have uncovered the 3D structure of RBP3, a key protein in vision, revealing how it transports retinoids and fatty acids and how its dysfunction may lead to retinal diseases. Proteins play a critical [...]
5 Key Facts About Nanoplastics and How They Affect the Human Body
Nanoplastics are typically defined as plastic particles smaller than 1000 nanometers. These particles are increasingly being detected in human tissues: they can bypass biological barriers, accumulate in organs, and may influence health in ways [...]
Measles Is Back: Doctors Warn of Dangerous Surge Across the U.S.
Parents are encouraged to contact their pediatrician if their child has been exposed to measles or is showing symptoms. Pediatric infectious disease experts are emphasizing the critical importance of measles vaccination, as the highly [...]
AI at the Speed of Light: How Silicon Photonics Are Reinventing Hardware
A cutting-edge AI acceleration platform powered by light rather than electricity could revolutionize how AI is trained and deployed. Using photonic integrated circuits made from advanced III-V semiconductors, researchers have developed a system that vastly [...]
A Grain of Brain, 523 Million Synapses, Most Complicated Neuroscience Experiment Ever Attempted
A team of over 150 scientists has achieved what once seemed impossible: a complete wiring and activity map of a tiny section of a mammalian brain. This feat, part of the MICrONS Project, rivals [...]
The Secret “Radar” Bacteria Use To Outsmart Their Enemies
A chemical radar allows bacteria to sense and eliminate predators. Investigating how microorganisms communicate deepens our understanding of the complex ecological interactions that shape our environment is an area of key focus for the [...]
Psychologists explore ethical issues associated with human-AI relationships
It's becoming increasingly commonplace for people to develop intimate, long-term relationships with artificial intelligence (AI) technologies. At their extreme, people have "married" their AI companions in non-legally binding ceremonies, and at least two people [...]
When You Lose Weight, Where Does It Actually Go?
Most health professionals lack a clear understanding of how body fat is lost, often subscribing to misconceptions like fat converting to energy or muscle. The truth is, fat is actually broken down into carbon [...]
How Everyday Plastics Quietly Turn Into DNA-Damaging Nanoparticles
The same unique structure that makes plastic so versatile also makes it susceptible to breaking down into harmful micro- and nanoscale particles. The world is saturated with trillions of microscopic and nanoscopic plastic particles, some smaller [...]
AI Outperforms Physicians in Real-World Urgent Care Decisions, Study Finds
The study, conducted at the virtual urgent care clinic Cedars-Sinai Connect in LA, compared recommendations given in about 500 visits of adult patients with relatively common symptoms – respiratory, urinary, eye, vaginal and dental. [...]