
Ocular Anomalies
Microgravity induced ophthalmic anomalies were observed by Mader et al in seven astronauts who were involved in long-duration (six month) space missions to the ISS. An additional 300 astronauts were asked to complete a questionnaire in regard to in-flight vision changes. For the seven astronauts under study, the ophthalmic results indicated disk edema (in five), globe flattening (in five), choroidal folds (in five), cotton wool spots (in three), nerve fiber layer thickening (in six), decreased near-vision (in six). Further, optic nerve sheath distension and tortuous optic nerves were observed.
Of the 300 questionnaire respondents, 60% of the astronauts who were engaged in long duration missions reported experiencing degradation in both their near and distance vision; these conditions persisted for years postflight for some individuals. The researchers concluded that, “the optic nerve and ocular changes we describe may result from cephalad
[toward the head] fluid shifts brought about by prolonged microgravity exposure. The findings we report may represent parts of a spectrum of ocular and cerebral responses to extended microgravity exposure.”
Several sets of mice were included aboard shuttle flights STS-133 and STS-135 (March and July 2011), respectively, as a component of the Commercial Biomedical Testing Module-2 (CBTM-2) and CBTM-3, which investigated the impacts of space on muscle tissues and bones. These dual studies were the first to provide direct evidence, subsequent to the examination/imaging of the eye tissues of the mice (Figure 1) that spaceflight can initiate cellular injury with the capacity to cause long-term vision issues.
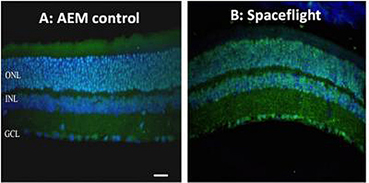
Figure 1: Retinal tissue from normal mouse (A) and that following spaceflight (B) reveal oxidative damage (green fluorescence). Blue stained entities are nuclei. (Image credit: Radiation Research)
Nanomedical Strategies to Alleviate Microgravity-Induced Ocular Anomalies
The degradative impacts of microgravity itself (as distinct from ionizing radiation) on the cells of the eye require further elucidation. A NASA-designed rotating wall vessel (RWV) bioreactor was employed by Roberts et al to simulate microgravity to assess its effects on human retinal pigment epithelial (hRPE) cells, in contrast to controls in a non-rotating wall vessel under ambient gravity. It was observed that following 24 hours of simulated microgravity, the hRPE cells showed a significant level of oligonucleotide (single-stranded DNA) breaks when compared with the controls, which were not resolved 48 hours post exposure.
However, the numbers of breaks were halved when the cells were pretreated with 1 μM cysteine, followed by a 48 h recuperation period. Further, a significant secretion of prostaglandin E2 (PGE2) was observed, which implied an acute microgravity induced inflammatory response. Retinal inflammation is of serious concern for astronauts, and immune cells that manage to breach the blood retina barrier (BRB) may initiate an immune response that results in retinal deterioration and/or retinal detachment.
A potential nanomedical strategy to address retinal inflammation might involve the targeted delivery of lutein (Figure 2) and zeaxanthin-loaded within nanocarriers (e.g., liposomes, dendrimers, and albumin) (Figures 3a-c) that can transit through the BRB without cytotoxicity, to directly deliver their payloads to the retina to preserve its functionality. Lutein, zeaxanthin, and meso-zeaxanthin are xanthophylls carotenoids are collectively known as the macular pigment. Together they can protect retinal neurons by serving as antioxidants, anti-apoptotics, and anti-inflammatory agents.
Figure 2: Graphic of lutein molecule
Figure 3a Liposome (Image credit: lipolife)
Figure 3b Dendrimer (Image credit: Wikimedia commons)
Figure 3c Albumin Image credit: (PKD Diet)
Cerium oxide nanoparticles (Figure 4) can act as regenerative catalytic antioxidants to protect neurons of the retina because of their redox (reduction–oxidation reaction) capacities. It was revealed by Deshpande et al that as the dimensions of ceria nanoparticles are reduced (~Ø3–5 nm), the population of available vacant sites for oxygen atoms within its crystalline structure is enhanced.
Figure 4: Ceria oxide nanoparticle Image credit: (Wikipedia)