The receptor sticks to the virus and pulls it away from the target cells
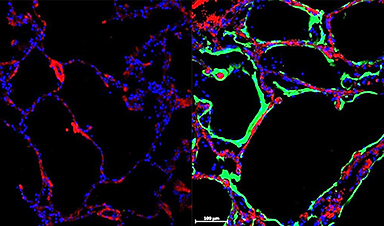
![Left: Control Lung. Right: Immunofluorescent staining shows expression of new SARS-CoV-2 spike-receptor LRRC15 (green) in post-mortem lung tissue section from individual with COVID-19 [Credit: Loo and Waller et al.]](https://www.sydney.edu.au/dam/corporate/images/news-and-opinion/news/2023/february/receptor-image.jpg/_jcr_content/renditions/cq5dam.web.1280.1280.jpeg)
Left: Control Lung. Right: Immunofluorescent staining shows expression of new SARS-CoV-2 spike-receptor LRRC15 (green) in post-mortem lung tissue section from individual with COVID-19 [Credit: Loo and Waller et al.]
This protein, the leucine-rich repeat-containing protein 15 (LRRC15), is an inbuilt receptor that binds the SARS-CoV-2 virus without passing on the infection.
The research opens up an entirely new area of immunology research around LRRC15 and offers a promising pathway to develop new drugs to prevent viral infection from coronaviruses like COVID-19 or deal with fibrosis in the lungs.
The study has been published in the journal PLOS Biology. It was led by Professor Greg Neely with his team members Dr Lipin Loo, a postdoctoral researcher, and PhD student Matthew Waller at the Charles Perkins Centre and the School of Life and Environmental Sciences.
The University study is one of three independent papers that reveal this specific protein’s interaction with COVID-19.
“Alongside two other groups, one at Oxford, the other at Brown and Yale in the USA, we found a new receptor in the LRRC15 protein that can stop SARS-CoV-2. We found that this new receptor acts by binding to the virus and sequestering it which reduces infection,” Professor Neely said.
“For me, as an immunologist, the fact that there’s this natural immune receptor that we didn’t know about, that’s lining our lungs and blocks and controls virus, that’s crazy interesting.
“We can now use this new receptor to design broad acting drugs that can block viral infection or even suppress lung fibrosis.”
What is LRRC15?
The COVID-19 virus infects humans by using a spike protein to attach to a specific receptor in our cells. It primarily uses a protein called the angiotensin-converting enzyme 2 (ACE2) receptor to enter human cells. Lung cells have high levels of ACE2 receptors, which is why the COVID-19 virus often causes severe problems in this organ of infected people.
Like ACE2, LRRC15 is a receptor for coronavirus, meaning the virus can bind to it. But unlike ACE2, LRRC15 does not support infection. It can, however, stick to the virus and immobilise it. In the process, it prevents other vulnerable cells from becoming infected.
“We think it acts a bit like Velcro, molecular Velcro, in that it sticks to the spike of the virus and then pulls it away from the target cell types,” Dr Loo said.
“Basically, the virus is coated in the other part of the Velcro, and while it’s trying to get to the main receptor, it can get caught up in this mesh of LRRC15,” Mr Waller said.
LRRC15 is present in many locations such as lungs, skin, tongue, fibroblasts, placenta and lymph nodes. But the researchers found human lungs light up with LRRC15 after infection.
“When we stain the lungs of healthy tissue, we don’t see much of LRRC15, but then in COVID-19 lungs, we see much more of the protein,” Dr Loo said.
“We think this newly identified protein could be part of our body’s natural response to combating the infection creating a barrier that physically separates the virus from our lung cells most sensitive to COVID-19.”
![Pastel pop art illustration of human lung generated using OpenAI’s DALL·E 2 [Credit: Greg Neely]](https://www.sydney.edu.au/dam/corporate/images/news-and-opinion/news/2023/february/image-2.jpeg/_jcr_content/renditions/cq5dam.web.1280.1280.jpeg)
Pastel pop art illustration of human lung generated using OpenAI’s DALL·E 2 [Credit: Greg Neely]
Implications of the research
“When we studied how this new receptor works, we found that this receptor also controls antiviral responses, as well as fibrosis, and could link COVID-19 infection with lung fibrosis that occurs during long COVID,” Mr Waller said.
“Since this receptor can block COVID-19 infection, and at the same time activate our body’s anti-virus response, and suppress our body’s fibrosis response, this is a really important new gene,” Professor Neely said.
“This finding can help us develop new antiviral and antifibrotic medicines to help treat pathogenic coronaviruses, and possibly other viruses or other situations where lung fibrosis occurs.
“For fibrosis, there are no good drugs: for example, idiopathic pulmonary fibrosis is currently untreatable.”
Fibrosis is a condition in which lung tissue becomes scarred and thickened, causing breathing difficulties. COVID-19 can cause inflammation and damage to the lungs, leading to fibrosis.
The authors said they are developing two strategies against COVID-19 using LRRC15 that could work across multiple variants – one which targets the nose as a preventative treatment, and another aimed at the lungs for serious cases.
The researchers also said that the presence or lack of LRRC15, which is involved in lung repair, is an important indication of how severe a COVID-19 infection might become.
“A group at Imperial College London independently found that absence of LRRC15 in the blood is associated with more severe COVID, which supports what we think is happening.” Dr Loo said. “If you have less of this protein, you likely have serious COVID. If you have more of it, your COVID is less severe.
“We are now trying to understand exactly why this is the case.”
The research involved screening human cell cultures for genes and investigating the lungs of human COVID-19 patients.
News
Baffling Scientists for Centuries: New Study Unravels Mystery of Static Electricity
ISTA physicists demonstrate that contact electrification depends on the contact history of materials. For centuries, static electricity has intrigued and perplexed scientists. Now, researchers from the Waitukaitis group at the Institute of Science and [...]
Tumor “Stickiness” – Scientists Develop Potential New Way To Predict Cancer’s Spread
UC San Diego researchers have developed a device that predicts breast cancer aggressiveness by measuring tumor cell adhesion. Weakly adherent cells indicate a higher risk of metastasis, especially in early-stage DCIS. This innovation could [...]
Scientists Just Watched Atoms Move for the First Time Using AI
Scientists have developed a groundbreaking AI-driven technique that reveals the hidden movements of nanoparticles, essential in materials science, pharmaceuticals, and electronics. By integrating artificial intelligence with electron microscopy, researchers can now visualize atomic-level changes that were [...]
Scientists Sound Alarm: “Safe” Antibiotic Has Led to an Almost Untreatable Superbug
A recent study reveals that an antibiotic used for liver disease patients may increase their risk of contracting a dangerous superbug. An international team of researchers has discovered that rifaximin, a commonly prescribed antibiotic [...]
Scientists Discover Natural Compound That Stops Cancer Progression
A discovery led by OHSU was made possible by years of study conducted by University of Portland undergraduates. Scientists have discovered a natural compound that can halt a key process involved in the progression [...]
Scientists Just Discovered an RNA That Repairs DNA Damage – And It’s a Game-Changer
Our DNA is constantly under threat — from cell division errors to external factors like sunlight and smoking. Fortunately, cells have intricate repair mechanisms to counteract this damage. Scientists have uncovered a surprising role played by [...]
What Scientists Just Discovered About COVID-19’s Hidden Death Toll
COVID-19 didn’t just claim lives directly—it reshaped mortality patterns worldwide. A major international study found that life expectancy plummeted across most of the 24 analyzed countries, with additional deaths from cardiovascular disease, substance abuse, and mental [...]
Self-Propelled Nanoparticles Improve Immunotherapy for Non-Invasive Bladder Cancer
A study led by Pohang University of Science and Technology (POSTECH) and the Institute for Bioengineering of Catalonia (IBEC) in South Korea details the creation of urea-powered nanomotors that enhance immunotherapy for bladder cancer. The nanomotors [...]
Scientists Develop New System That Produces Drinking Water From Thin Air
UT Austin researchers have developed a biodegradable, biomass-based hydrogel that efficiently extracts drinkable water from the air, offering a scalable, sustainable solution for water access in off-grid communities, emergency relief, and agriculture. Discarded food [...]
AI Unveils Hidden Nanoparticles – A Breakthrough in Early Disease Detection
Deep Nanometry (DNM) is an innovative technique combining high-speed optical detection with AI-driven noise reduction, allowing researchers to find rare nanoparticles like extracellular vesicles (EVs). Since EVs play a role in disease detection, DNM [...]
Inhalable nanoparticles could help treat chronic lung disease
Nanoparticles designed to release antibiotics deep inside the lungs reduced inflammation and improved lung function in mice with symptoms of chronic obstructive pulmonary disease By Grace Wade Delivering medication to the lungs with inhalable nanoparticles [...]
New MRI Study Uncovers Hidden Lung Abnormalities in Children With Long COVID
Long COVID is more than just lingering symptoms—it may have a hidden biological basis that standard medical tests fail to detect. A groundbreaking study using advanced MRI technology has uncovered significant lung abnormalities in [...]
AI Struggles with Abstract Thought: Study Reveals GPT-4’s Limits
While GPT-4 performs well in structured reasoning tasks, a new study shows that its ability to adapt to variations is weak—suggesting AI still lacks true abstract understanding and flexibility in decision-making. Artificial Intelligence (AI), [...]
Turning Off Nerve Signals: Scientists Develop Promising New Pancreatic Cancer Treatment
Pancreatic cancer reprograms nerve cells to fuel its growth, but blocking these connections can shrink tumors and boost treatment effectiveness. Pancreatic cancer is closely linked to the nervous system, according to researchers from the [...]
New human antibody shows promise for Ebola virus treatment
New research led by scientists at La Jolla Institute for Immunology (LJI) reveals the workings of a human antibody called mAb 3A6, which may prove to be an important component for Ebola virus therapeutics. [...]
Early Alzheimer’s Detection Test – Years Before Symptoms Appear
A new biomarker test can detect early-stage tau protein clumping up to a decade before it appears on brain scans, improving early Alzheimer’s diagnosis. Unlike amyloid-beta, tau neurofibrillary tangles are directly linked to cognitive decline. Years [...]