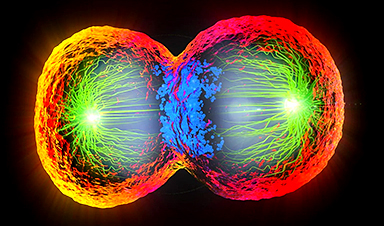
Scientists at EMBL have captured how human chromosomes fold into their signature rod shape during cell division, using a groundbreaking method called LoopTrace.
By observing overlapping DNA loops forming in high resolution, they revealed that large loops form first, followed by nested smaller loops, all repelling each other into compact structures. This new insight not only reshapes our understanding of chromosome mechanics but could also help explain errors that lead to cancer and genetic disorders.
The Mystery of Chromosome Division
One of the most remarkable abilities of living cells is their capacity to divide, allowing organisms to grow, heal, and renew themselves. To do this, a cell must first make an exact copy of its DNA, its genome, and ensure each daughter cell receives a complete set.
In humans, that means carefully packaging 46 chromosomes and distributing them equally. Before division, each chromosome transforms into a compact, X-shaped structure made of two identical, rod-like copies. But exactly how cells manage to reshape and organize their DNA for this process has remained a mystery.
Now, for the first time, scientists at EMBL have directly visualized this process in high resolution using a new chromatin tracing technique. Their study reveals that during cell division, the long strands of DNA form a series of overlapping loops that push away from one another. This repulsion causes the loops to stack, ultimately giving each chromosome its characteristic rod-like shape.

Looping DNA to Shape Chromosomes
Scientists have long hypothesized the importance of DNA loops in building and maintaining chromosomal structure. First identified in the 1990s, condensins are large protein complexes that bind DNA during cell division and extrude it to create loops of varying sizes. Previous studies from EMBL have shed light on the structural mechanics of this process and their essential role in packing chromosomes into forms that can be easily moved between cells.
In fact, mutations in condensin structure can result in severe chromosome segregation defects and lead to cell death, cancer formation, or rare developmental disorders called 'condensinopathies'.
Solving the DNA Imaging Problem
"However, observing how this looping process occurs on the cellular scale and contributes to chromosome structure is challenging," said Andreas Brunner, postdoc in EMBL Heidelberg's Ellenberg Group and a lead author of the new paper. "This is because methods for visualizing DNA with high resolution are usually chemically harsh and require high temperatures, which together disrupt the native structure of DNA."
Kai Beckwith, a former postdoc in the Ellenberg Group and currently an associate professor at the Norwegian University of Science and Technology (NTNU), set out to solve this problem. Beckwith and colleagues used a method to gently remove one strand of DNA in cells at various stages of cell division, keeping the chromosome structure intact. They could then use targeted sets of DNA-binding labels to observe the nanoscale organization of this uncovered DNA strand. This technique, called LoopTrace, helped the researchers directly observe DNA in dividing cells as it progressively formed loops and folds.
"Andreas and I were now able to visualize the structure of chromosomes as they started to change shape," said Beckwith. "This was crucial for understanding how the DNA was folded by the condensin complexes."
Nested Loops and DNA Compaction
From their data, the scientists realized that during cell division, DNA forms loops in two stages. First, it forms stable large loops, which then subdivide into smaller, short-lived nested loops, increasing the compaction at each stage. Two types of condensin protein complexes enable this process.
To understand how this looping eventually gives rise to rod-shaped chromosomes, the researchers built a computational model based on two simple assumptions. First, as observed, DNA forms overlapping loops – first large and then small – across its length with the help of Condensins. Second, these loops repel each other due to their structure and the chemistry of DNA. When the scientists fed these two assumptions into their model, they found that this was sufficient to give rise to a rod-shaped chromosome structure.
Overlapping Loops Are Key
"We realized that these condensin-driven loops are much larger than previously thought, and that it was very important that the large loops overlap to a significant extent," said Beckwith. "Only these features allowed us to recapitulate the native structure of mitotic chromosomes in our model and understand how they can be segregated during cell division."
In the future, the researchers plan to study this process in more detail, especially to understand how additional factors, such as molecular regulators, affect this compaction process. In 2024, Jan Ellenberg and his team received funding of €3.1 million (~$3.4 million) as an ERC Advanced Grant, to study the folding principles of chromosomes during and following cell division.
A Milestone for Chromosome Biology
"Our newest paper published in the scientific journal Cell marks a milestone in our understanding of how the cell is able to pack chromosomes for their accurate segregation into daughter cells," said Jan Ellenberg, Senior Scientist at EMBL Heidelberg. "It will be the basis to understand the molecular mechanism of rescaling the genome for faithful inheritance and thus rationally predict how errors in this process that underlie human disease could be prevented in the future."
In the meantime, a second study from the Ellenberg Team, led by Andreas Brunner and recently published in the Journal of Cell Biology, shows that the nested loop mechanism is fundamental to the biology of cells, and continues during the cell's growth phase with another family of DNA loop forming protein complexes, called cohesins.
Looping Mechanisms Across Cell Phases
"We were surprised to find that the same core principle of sequential and hierarchical DNA loop formation is used to either tightly pack chromosomes during division into safely movable entities, or to unpack them afterward to read out the information they contain," said Ellenberg. "In the end, small, but key mechanistic differences, such as the non-overlapping nature of cohesin-driven loops compared to the strongly overlapping condensin-driven loops might be sufficient to explain the vast differences that we see in the shape the genome takes in interphase and mitosis under the microscope."
References:
Reference: "Nanoscale DNA tracing reveals the self-organization mechanism of mitotic chromosomes" by Kai Sandvold Beckwith, Andreas Brunner, Natalia Rosalia Morero, Ralf Jungmann and Jan Ellenberg, 24 March 2025, Cell.
DOI: 10.1016/j.cell.2025.02.028
"Quantitative imaging of loop extruders rebuilding interphase genome architecture after mitosis" by Andreas Brunner, Natalia Rosalía Morero, Wanlu Zhang, M. Julius Hossain, Marko Lampe, Hannah Pflaumer, Aliaksandr Halavatyi, Jan-Michael Peters, Kai S. Beckwith and Jan Ellenberg, 9 January 2025, Journal of Cell Biology.
DOI: 10.1083/jcb.202405169
News
How lipid nanoparticles carrying vaccines release their cargo
A study from FAU has shown that lipid nanoparticles restructure their membrane significantly after being absorbed into a cell and ending up in an acidic environment. Vaccines and other medicines are often packed in [...]
New book from NanoappsMedical Inc – Molecular Manufacturing: The Future of Nanomedicine
This book explores the revolutionary potential of atomically precise manufacturing technologies to transform global healthcare, as well as practically every other sector across society. This forward-thinking volume examines how envisaged Factory@Home systems might enable the cost-effective [...]
A Virus Designed in the Lab Could Help Defeat Antibiotic Resistance
Scientists can now design bacteria-killing viruses from DNA, opening a faster path to fighting superbugs. Bacteriophages have been used as treatments for bacterial infections for more than a century. Interest in these viruses is rising [...]
Sleep Deprivation Triggers a Strange Brain Cleanup
When you don’t sleep enough, your brain may clean itself at the exact moment you need it to think. Most people recognize the sensation. After a night of inadequate sleep, staying focused becomes harder [...]
Lab-grown corticospinal neurons offer new models for ALS and spinal injuries
Researchers have developed a way to grow a highly specialized subset of brain nerve cells that are involved in motor neuron disease and damaged in spinal injuries. Their study, published today in eLife as the final [...]
Urgent warning over deadly ‘brain swelling’ virus amid fears it could spread globally
Airports across Asia have been put on high alert after India confirmed two cases of the deadly Nipah virus in the state of West Bengal over the past month. Thailand, Nepal and Vietnam are among the [...]
This Vaccine Stops Bird Flu Before It Reaches the Lungs
A new nasal spray vaccine could stop bird flu at the door — blocking infection, reducing spread, and helping head off the next pandemic. Since first appearing in the United States in 2014, H5N1 [...]
These two viruses may become the next public health threats, scientists say
Two emerging pathogens with animal origins—influenza D virus and canine coronavirus—have so far been quietly flying under the radar, but researchers warn conditions are ripe for the viruses to spread more widely among humans. [...]
COVID-19 viral fragments shown to target and kill specific immune cells
COVID-19 viral fragments shown to target and kill specific immune cells in UCLA-led study Clues about extreme cases and omicron’s effects come from a cross-disciplinary international research team New research shows that after the [...]
Smaller Than a Grain of Salt: Engineers Create the World’s Tiniest Wireless Brain Implant
A salt-grain-sized neural implant can record and transmit brain activity wirelessly for extended periods. Researchers at Cornell University, working with collaborators, have created an extremely small neural implant that can sit on a grain of [...]
Scientists Develop a New Way To See Inside the Human Body Using 3D Color Imaging
A newly developed imaging method blends ultrasound and photoacoustics to capture both tissue structure and blood-vessel function in 3D. By blending two powerful imaging methods, researchers from Caltech and USC have developed a new way to [...]
Brain waves could help paralyzed patients move again
People with spinal cord injuries often lose the ability to move their arms or legs. In many cases, the nerves in the limbs remain healthy, and the brain continues to function normally. The loss of [...]
Scientists Discover a New “Cleanup Hub” Inside the Human Brain
A newly identified lymphatic drainage pathway along the middle meningeal artery reveals how the human brain clears waste. How does the brain clear away waste? This task is handled by the brain’s lymphatic drainage [...]
New Drug Slashes Dangerous Blood Fats by Nearly 40% in First Human Trial
Scientists have found a way to fine-tune a central fat-control pathway in the liver, reducing harmful blood triglycerides while preserving beneficial cholesterol functions. When we eat, the body turns surplus calories into molecules called [...]
A Simple Brain Scan May Help Restore Movement After Paralysis
A brain cap and smart algorithms may one day help paralyzed patients turn thought into movement—no surgery required. People with spinal cord injuries often experience partial or complete loss of movement in their arms [...]
Plant Discovery Could Transform How Medicines Are Made
Scientists have uncovered an unexpected way plants make powerful chemicals, revealing hidden biological connections that could transform how medicines are discovered and produced. Plants produce protective chemicals called alkaloids as part of their natural [...]