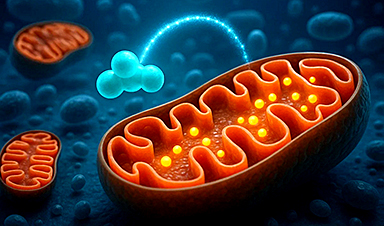
After more than five decades of mystery, scientists have finally unveiled the detailed structure and function of a long-theorized molecular machine in our mitochondria — the mitochondrial pyruvate carrier.
This microscopic gatekeeper controls how cells fuel themselves by transporting pyruvate, a key energy source, across mitochondrial membranes. Now visualized using cryo-electron microscopy, the carrier’s lock-like mechanism could be the key to tackling diseases like cancer, diabetes, and even hair loss. By blocking or modifying this gateway, researchers believe we could reroute how cells generate energy and develop powerful, targeted treatments.
Unlocking a Mitochondrial Mystery
After more than 50 years, scientists have finally uncovered how a tiny molecular machine inside our cells helps turn sugar into energy, a process essential for life.
Researchers at the Medical Research Council (MRC) Mitochondrial Biology Unit at the University of Cambridge have revealed the structure of this machine, which works like a canal lock to move a molecule called pyruvate into the mitochondria — the parts of our cells often called the “powerhouses.” Pyruvate is produced when our bodies break down sugars, and it plays a key role in energy production.
Visualizing the Invisible
This machine, known as the mitochondrial pyruvate carrier, was first proposed in 1971. But only now have scientists been able to visualize it at the atomic level, using a powerful imaging technique called cryo-electron microscopy, which magnifies structures up to 165,000 times their size. The findings appear today (April 18) in Science Advances.
Dr. Sotiria Tavoulari, Senior Research Associate at the University of Cambridge, who helped identify the components of the carrier, explained: “Sugars in our diet provide energy for our bodies to function. When they are broken down inside our cells they produce pyruvate, but to get the most out of this molecule, it needs to be transferred inside the cell’s powerhouses, the mitochondria. There, it helps increase 15-fold the energy produced in the form of the cellular fuel ATP.”
Revealing the Transport Mechanism
Maximilian Sichrovsky, a PhD student at Hughes Hall and joint first author of the study, said: “Getting pyruvate into our mitochondria sounds straightforward, but until now we haven’t been able to understand the mechanism of how this process occurs. Using state-of-the-art cryo-electron microscopy, we’ve been able to show not only what this transporter looks like, but exactly how it works. It’s an extremely important process, and understanding it could lead to new treatments for a range of different conditions.”
Molecular Locks and Canal Gates
Mitochondria are surrounded by two membranes. The outer one is porous, and pyruvate can easily pass through, but the inner membrane is impermeable to pyruvate. To transport pyruvate into the mitochondrion, first an outer ‘gate’ of the carrier opens, allowing pyruvate to enter the carrier. This gate then closes, and the inner gate opens, allowing the molecule to pass through into the mitochondrion.
“It works like the locks on a canal but on the molecular scale,” said Professor Edmund Kunji from the MRC Mitochondrial Biology Unit, and a Fellow at Trinity Hall, Cambridge. “There, a gate opens at one end, allowing the boat to enter. It then closes, and the gate at the opposite end opens to allow the boat smooth transit through.”
A New Drug Target Emerges
Because of its central role in controlling the way mitochondria operate to produce energy, this carrier is now recognised as a promising drug target for a range of conditions, including diabetes, fatty liver disease, Parkinson’s disease, specific cancers, and even hair loss.
Pyruvate is not the only energy source available to us. Our cells can also take their energy from fats stored in the body or from amino acids in proteins. Blocking the pyruvate carrier would force the body to look elsewhere for its fuel – creating opportunities to treat a number of diseases. In fatty liver disease, for example, blocking access to pyruvate entry into mitochondria could encourage the body to use potentially dangerous fat that has been stored in liver cells.
Starving Cancer and Stimulating Hair Growth
Likewise, there are certain tumour cells that rely on pyruvate metabolism, such as in some types of prostate cancer. These cancers tend to be very ‘hungry’, producing excess pyruvate transport carriers to ensure they can feed more. Blocking the carrier could then starve these cancer cells of the energy they need to survive, killing them.
Previous studies have also suggested that inhibiting the mitochondrial pyruvate carrier may reverse hair loss. Activation of human follicle cells, which are responsible for hair growth, relies on metabolism and, in particular, the generation of lactate. When the mitochondrial pyruvate carrier is blocked from entering the mitochondria in these cells, it is instead converted to lactate.
Drug Design Gets a Molecular Blueprint
Professor Kunji said: “Drugs inhibiting the function of the carrier can remodel how mitochondria work, which can be beneficial in certain conditions. Electron microscopy allows us to visualize exactly how these drugs bind inside the carrier to jam it – a spanner in the works, you could say. This creates new opportunities for structure-based drug design in order to develop better, more targeted drugs. This will be a real game changer.”
Reference: “Molecular basis of pyruvate transport and inhibition of the human mitochondrial pyruvate carrier” by Sichrovsky, M, Lacabanne, D, Ruprecht, JJ & Rana, JJ et al., 18 April 2025, Science Advances.
DOI: 10.1126/sciadv.adw1489
The research was supported by the Medical Research Council and was a collaboration with the groups of Professors Vanessa Leone at the Medical College of Wisconsin, Lucy Forrest at the National Institutes of Health, and Jan Steyaert at the Free University of Brussels.
News
This Tiny Cellular Gate Could Be the Key to Curing Cancer – And Regrowing Hair
After more than five decades of mystery, scientists have finally unveiled the detailed structure and function of a long-theorized molecular machine in our mitochondria — the mitochondrial pyruvate carrier. This microscopic gatekeeper controls how [...]
Unlocking Vision’s Secrets: Researchers Reveal 3D Structure of Key Eye Protein
Researchers have uncovered the 3D structure of RBP3, a key protein in vision, revealing how it transports retinoids and fatty acids and how its dysfunction may lead to retinal diseases. Proteins play a critical [...]
5 Key Facts About Nanoplastics and How They Affect the Human Body
Nanoplastics are typically defined as plastic particles smaller than 1000 nanometers. These particles are increasingly being detected in human tissues: they can bypass biological barriers, accumulate in organs, and may influence health in ways [...]
Measles Is Back: Doctors Warn of Dangerous Surge Across the U.S.
Parents are encouraged to contact their pediatrician if their child has been exposed to measles or is showing symptoms. Pediatric infectious disease experts are emphasizing the critical importance of measles vaccination, as the highly [...]
AI at the Speed of Light: How Silicon Photonics Are Reinventing Hardware
A cutting-edge AI acceleration platform powered by light rather than electricity could revolutionize how AI is trained and deployed. Using photonic integrated circuits made from advanced III-V semiconductors, researchers have developed a system that vastly [...]
A Grain of Brain, 523 Million Synapses, Most Complicated Neuroscience Experiment Ever Attempted
A team of over 150 scientists has achieved what once seemed impossible: a complete wiring and activity map of a tiny section of a mammalian brain. This feat, part of the MICrONS Project, rivals [...]
The Secret “Radar” Bacteria Use To Outsmart Their Enemies
A chemical radar allows bacteria to sense and eliminate predators. Investigating how microorganisms communicate deepens our understanding of the complex ecological interactions that shape our environment is an area of key focus for the [...]
Psychologists explore ethical issues associated with human-AI relationships
It's becoming increasingly commonplace for people to develop intimate, long-term relationships with artificial intelligence (AI) technologies. At their extreme, people have "married" their AI companions in non-legally binding ceremonies, and at least two people [...]
When You Lose Weight, Where Does It Actually Go?
Most health professionals lack a clear understanding of how body fat is lost, often subscribing to misconceptions like fat converting to energy or muscle. The truth is, fat is actually broken down into carbon [...]
How Everyday Plastics Quietly Turn Into DNA-Damaging Nanoparticles
The same unique structure that makes plastic so versatile also makes it susceptible to breaking down into harmful micro- and nanoscale particles. The world is saturated with trillions of microscopic and nanoscopic plastic particles, some smaller [...]
AI Outperforms Physicians in Real-World Urgent Care Decisions, Study Finds
The study, conducted at the virtual urgent care clinic Cedars-Sinai Connect in LA, compared recommendations given in about 500 visits of adult patients with relatively common symptoms – respiratory, urinary, eye, vaginal and dental. [...]
Challenging the Big Bang: A Multi-Singularity Origin for the Universe
In a study published in the journal Classical and Quantum Gravity, Dr. Richard Lieu, a physics professor at The University of Alabama in Huntsville (UAH), which is a part of The University of Alabama System, suggests that [...]
New drug restores vision by regenerating retinal nerves
Vision is one of the most crucial human senses, yet over 300 million people worldwide are at risk of vision loss due to various retinal diseases. While recent advancements in retinal disease treatments have [...]
Shingles vaccine cuts dementia risk by 20%, new study shows
A shingles shot may do more than prevent rash — it could help shield the aging brain from dementia, according to a landmark study using real-world data from the UK. A routine vaccine could [...]
AI Predicts Sudden Cardiac Arrest Days Before It Strikes
AI can now predict deadly heart arrhythmias up to two weeks in advance, potentially transforming cardiac care. Artificial intelligence could play a key role in preventing many cases of sudden cardiac death, according to [...]
NanoApps Medical is a Top 20 Feedspot Nanotech Blog
There is an ocean of Nanotechnology news published every day. Feedspot saves us a lot of time and we recommend it. We have been using it since 2018. Feedspot is a freemium online RSS [...]