New materials with near-perfect water repellency offer potential for self-cleaning surfaces in cars and buildings.
Scientists from Karlsruhe Institute of Technology (KIT) and the Indian Institute of Technology Guwahati (IITG) have developed a surface material that repels water droplets almost completely. Using an entirely innovative process, they changed metal-organic frameworks (MOFs) – artificially designed materials with novel properties – by grafting hydrocarbon chains. The resulting superhydrophobic (extremely water-repellent) properties are interesting for use as self-cleaning surfaces that need to be robust against environmental influences, such as on automobiles or in architecture. The study was published in the Materials Horizons journal.
Superhydrophobic Surfaces from MOFs
Metal-organic frameworks (MOFs) are made of metal ions connected by organic linkers, forming a porous, sponge-like structure. Their remarkable surface area — just two grams can cover the size of a football field — makes them valuable for applications like gas storage, carbon dioxide separation, and advanced medical technologies.
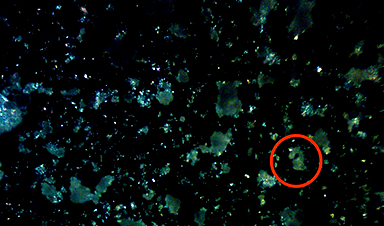
Beyond their internal pores, the outer surfaces of MOFs also offer unique properties. Researchers enhanced these surfaces by grafting hydrocarbon chains onto thin MOF films, creating a water-repellent material with a contact angle of over 160 degrees. A higher contact angle means better hydrophobicity, as water droplets form a nearly spherical shape instead of spreading out.
“With our method, we are able to achieve superhydrophobic surfaces with contact angles that are significantly higher than those of other smooth surfaces and coatings,” explains Professor Christof Wöll from KIT’s Institute of Functional Interfaces. “Although the wetting properties of MOF powder particles have been explored before, the use of monolithic MOF thin films for this purpose is a groundbreaking concept.”

The team attributes these results to the brush-like arrangement (polymer brushes) of the hydrocarbon chains on the MOFs. After being grafted to the MOF materials, they tend to form “coils” – a state of disorder that scientists call “high-entropy state,” which is essential for its hydrophobic properties. The scientists asserted that this state of the grafted hydrocarbon chains could not be observed on other materials.
It is remarkable that the water contact angle did not increase even when they used perfluorinated hydrocarbon chains for grafting, i.e. substituting hydrogen atoms with fluorine. In materials such as Teflon, perfluorination brings about superhydrophobic properties. In the newly developed material, however, it decreased the water contact angle significantly, as the team found out. Further analyses in computer simulations confirmed that the perfluorinated molecules – in contrast to hydrocarbon chains – could not assume the energetically favorable high-entropy state.
Insights from Surface Roughness and Theoretical Analysis
In addition, the scientists varied the surface roughness of their SAM@SURMOF systems in the nanometer range, thereby further reducing the water adhesion strength. Even with extremely small inclination angles, water droplets started rolling off, and their hydrophobic and self-cleaning properties were significantly improved.
“Our work also includes a detailed theoretical analysis, which links the unexpected behavior shown in experiments to the high-entropy state of the molecules grafted to the MOF films,” says Professor Uttam Manna from IITG’s Chemistry department. “This study will change the design and production of next-generation materials with optimum hydrophobic properties.”
Reference: “Functionalization of monolithic MOF thin films with hydrocarbon chains to achieve superhydrophobic surfaces with tunable water adhesion strength” by Evgenia Bogdanova, Modan Liu, Patrick Hodapp, Angana Borbora, Wolfgang Wenzel, Stefan Bräse, André Jung, Zheqin Dong, Pavel A. Levkin, Uttam Manna, Tawheed Hashem and Christof Wöll, 15 November 2024, Materials Horizons.
DOI: 10.1039/D4MH00899E
News
How Everyday Plastics Quietly Turn Into DNA-Damaging Nanoparticles
The same unique structure that makes plastic so versatile also makes it susceptible to breaking down into harmful micro- and nanoscale particles. The world is saturated with trillions of microscopic and nanoscopic plastic particles, some smaller [...]
AI Outperforms Physicians in Real-World Urgent Care Decisions, Study Finds
The study, conducted at the virtual urgent care clinic Cedars-Sinai Connect in LA, compared recommendations given in about 500 visits of adult patients with relatively common symptoms – respiratory, urinary, eye, vaginal and dental. [...]
Challenging the Big Bang: A Multi-Singularity Origin for the Universe
In a study published in the journal Classical and Quantum Gravity, Dr. Richard Lieu, a physics professor at The University of Alabama in Huntsville (UAH), which is a part of The University of Alabama System, suggests that [...]
New drug restores vision by regenerating retinal nerves
Vision is one of the most crucial human senses, yet over 300 million people worldwide are at risk of vision loss due to various retinal diseases. While recent advancements in retinal disease treatments have [...]
Shingles vaccine cuts dementia risk by 20%, new study shows
A shingles shot may do more than prevent rash — it could help shield the aging brain from dementia, according to a landmark study using real-world data from the UK. A routine vaccine could [...]
AI Predicts Sudden Cardiac Arrest Days Before It Strikes
AI can now predict deadly heart arrhythmias up to two weeks in advance, potentially transforming cardiac care. Artificial intelligence could play a key role in preventing many cases of sudden cardiac death, according to [...]
NanoApps Medical is a Top 20 Feedspot Nanotech Blog
There is an ocean of Nanotechnology news published every day. Feedspot saves us a lot of time and we recommend it. We have been using it since 2018. Feedspot is a freemium online RSS [...]
This Startup Says It Can Clean Your Blood of Microplastics
This is a non-exhaustive list of places microplastics have been found: Mount Everest, the Mariana Trench, Antarctic snow, clouds, plankton, turtles, whales, cattle, birds, tap water, beer, salt, human placentas, semen, breast milk, feces, testicles, [...]
New Blood Test Detects Alzheimer’s and Tracks Its Progression With 92% Accuracy
The new test could help identify which patients are most likely to benefit from new Alzheimer’s drugs. A newly developed blood test for Alzheimer’s disease not only helps confirm the presence of the condition but also [...]
The CDC buried a measles forecast that stressed the need for vaccinations
This story was originally published on ProPublica, a nonprofit newsroom that investigates abuses of power. Sign up to receive our biggest stories as soon as they’re published. ProPublica — Leaders at the Centers for Disease Control and Prevention [...]
Light-Driven Plasmonic Microrobots for Nanoparticle Manipulation
A recent study published in Nature Communications presents a new microrobotic platform designed to improve the precision and versatility of nanoparticle manipulation using light. Led by Jin Qin and colleagues, the research addresses limitations in traditional [...]
Cancer’s “Master Switch” Blocked for Good in Landmark Study
Researchers discovered peptides that permanently block a key cancer protein once thought untreatable, using a new screening method to test their effectiveness inside cells. For the first time, scientists have identified promising drug candidates [...]
AI self-cloning claims: A new frontier or a looming threat?
Chinese scientists claim that some AI models can replicate themselves and protect against shutdown. Has artificial intelligence crossed the so-called red line? Chinese researchers have published two reports on arXiv claiming that some artificial [...]
New Drug Turns Human Blood Into Mosquito-Killing Weapon
Nitisinone, a drug for rare diseases, kills mosquitoes when present in human blood and may become a new tool to fight malaria, offering longer-lasting, environmentally safer effects than ivermectin. Controlling mosquito populations is a [...]
DNA Microscopy Creates 3D Maps of Life From the Inside Out
What if you could take a picture of every gene inside a living organism—not with light, but with DNA itself? Scientists at the University of Chicago have pioneered a revolutionary imaging technique called volumetric DNA microscopy. It builds [...]
Scientists Just Captured the Stunning Process That Shapes Chromosomes
Scientists at EMBL have captured how human chromosomes fold into their signature rod shape during cell division, using a groundbreaking method called LoopTrace. By observing overlapping DNA loops forming in high resolution, they revealed that large [...]